
*
ATOMS
The third kingdom, that of atoms, introduces the possibility of identification, of location in space, of clear definition. Here we have the atom: it is carbon, or oxygen, or sodium; we can locate it, tag it, and know its properties. In fact, thanks to the same quantum theory that denies complete knowledge of the proton and electron in the second kingdom, we have a complete explanation for the atom in the third kingdom. Not only does every atom (or element) have properties that can be completely explained by reference to the number of
proton-electron pairs, called the atomic number, but the spectral lines radiated by the atom, which represent energy differences between orbits of electrons, can also be precisely accounted for.* The superbly rational properties of atoms are a characteristic we believe to be categorical, for, as we will later endeavor to show, the level of this kingdom (level III) is that at which concept formation occurs; more simply, it is form itself.
*The frequency of the spectral lines is predictable, but when the atom will radiate is unpredictable. This is the one degree of freedom to which we referred in our discussion of atoms in Chapter IV.
There are only about one hundred kinds of atom. I say "about" because those heavier than radium (atomic number 92) do not appear in nature, and, when created artificially, have lifetimes which become shorter and shorter as the weight increases, until it is only a fraction of a second. There is therefore an upper limit - hence a finite number of kinds of atom.
Rules for constructing atoms
We may note certain rules:
1. Atoms are constructed out of protons and electrons in equal numbers, or pairs - the heavy protons constituting the atom's nucleus, and the lighter electrons moving around it.
2. The number of proton-electron pairs determines the kind of atom. There is a different number for each atom (the atomic number) and a different atom for each number.
3. On account of the repulsion between positive protons in the nucleus, nuclei which contain more than one proton require an additional binding factor, or glue. This is provided by the addition of neutrons, which consist of an electron and a proton united together. There must be at least as many neutrons as protons for the nucleus to be stable.
We now encounter another important rule of atom-building. The added electrons fill up shells or rings, and the rule is that the first shell has two electrons which, when filled, is followed by another shell of two, then by a shell of six, then by another of six, then by a shell of ten, then another ten, then a shell of fourteen.
Angular momentum in the atom
It is the interpretation of these shells that has prompted the study of the atom. Because of them, the atom radiates and absorbs light of certain exact frequencies which show as lines in the spectrum (bright lines for radiation and dark lines for absorption). It was through deciphering these lines that the atom was understood.
Their position, which could be determined empirically with great accuracy, was not accounted for theoretically until Balmer, who was not a physicist but a numerologist, in 1885 discovered the formula for the lines of hydrogen which gave their exact value and even predicted other sets of lines:
![]()
But it was not until 1913 that Bohr, applying the concept of Planck that radiation occurs in quanta, gave an explanation of the behavior predicted by the formula (see Chapter II). Bohr assumed that the electron, like a vibrating violin string, can be in one of a number of discrete states, related by whole numbers, much as Pythagoras had found for the sound frequencies of a vibrating string. Bohr noted that when the electron jumps from one of these states to another, a quantum of energy in the form of a photon is emitted.
Then it was found that Bohr's explanation did not account for atoms with many electrons. Other explanations were advanced, and the quantum of action of Planck was found to be even more significant than had been suspected.
But the post-Bohr atom is not clearly explained in the textbooks. This is partly because so much was going on all at once. The de Broglie and Schroedinger wave equations and the discovery of the third and fourth quantum numbers (in addition to angular momentum and energy) overshadow what is important for the present theory: that in order for an electron to change orbits, the shape of the orbit has to change, and shape is due to angular momentum, not to energy. (See definition of angular momentum in the Additional Information section at the end of Chapter II.)
But why is shape of the orbit so important? Let us consider an atom in what is called the ground state. Here, the electron has no angular momentum. It is attracted to the nucleus like a moth to the flame, but its headlong dash toward the nucleus gives it such velocity that it is tossed back, like a comet passing the sun, as fast as it came, eventually to fall again, over and over. It cannot escape from the nucleus unless it receives a unit of angular momentum to give it circular motion and the option of choice instead of the in-and-out motion.

If we liken the electron to an astronaut in a spaceship, we can realize that this motion around the nucleus, like an orbit around the earth, represents a kind of power, a control of the situation that the same astronaut in free fall toward the earth would not have.
We can illustrate the situation in yet another way. Suppose a man raises cattle in Texas and wishes to transport them to Chicago for market. He can drive live cattle to market and sell them, and he's paid for the meat, but not for the aliveness, though it is the aliveness that got the cattle to Chicago. So it is with angular momentum, which can convey energy, but is not energy. It is the wholeness, the aliveness, which disappears when the creature is no longer whole. In the atom, it is orbit shape. Thus I am stressing that the aliveness (angular momentum or the whole) is more important than the physical energy (the part).
So we must not draw the conclusion that angular momentum is a mere "epiphenomenon," in the way that aliveness is regarded simply as the by-product of more basic causes. Here at atomic and subatomic levels we find it has an absolute value - h* - and it invariably possesses energy. While this energy is very small, it is not necessarily so; in cosmic rays it is quite large. But, in any case, this energy is the right amount for what it has to do; it is commensurate with the atoms and electrons whose state it changes.
*See discussion of Planck's constant, Chapter II.
In thus calling attention to angular momentum, I want not only to stress that angular momentum shows up as having a primary role in the world of the atom. I also want to indicate how the challenges of quantum physics have led science, quite against its first convictions, to new concepts that are different from its earlier rational materialistic ones. Here we have a direct revelation that energy, unless carried by angular momentum, cannot be transported from one atom to another.
The reason I say revelation is that, like revealed religion, it comes from a source that is not in the ordinary run of affairs, and like revealed religion, it has to be interpreted.
Above all, I stress that we must make the most of this clue, deciphered with such difficulty from the cryptic utterances of the atom, and apply it. For if it is true in the minute world of atoms, it could well be true in the larger world of people. But we need to inquire further into the nature of the atom.
The periodic table
This brings us to the periodic table of the elements, whose discovery is credited to Mendeleev (1869). By its help, Mendeleev was able to predict the properties of elements that had not then been discovered. He called it "periodic" because it showed the periodic recurrence of similar chemical properties. Even before this, Newlands, in 1863, suggested a table of seven rows and seven columns, which was an oversimplification, but neither Mendeleev nor Newlands was correct from the fourth row on, because it has been found that the 8-electron shells that were first proposed are actually composed of a 2- and a 6-electron subshell, to which are added, in the fourth and fifth rows, a 10-electron subshell, and in the sixth and seventh rows, a 14-electron subshell. The 10- and 14-shells are closest to the nucleus, buried under the 2- and 6-shells, and hence do not influence chemical properties. That is one reason why they were not taken into account by Mendeleev and Newlands.
For simplicity, let's look at the first three rows of the periodic table:
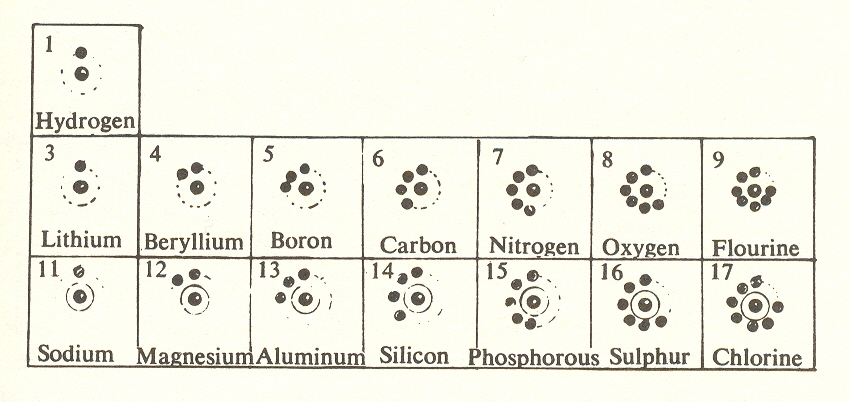
This is also the first three rows of Newland's table. With few exceptions, it includes the elements most important to the chemistry of life. In this table we can see the combining ratios, i.e., how to combine atoms in such proportions so there will be a total of eight electrons in a stable molecule.

Carbon, in the fourth column, combines with all the rest, including itself (diamond). These combining ratios hold good for elements of the same columns in all rows.
The combining ratios, however, deal only with the columns, and do not tell us about the rows. It is the rows that are the major divisions, and hence are the guide to the process of atomic evolution. We must therefore give consideration to the extra shells introduced in the fourth to seventh rows. By separating the added rows into blocks, it is possible to get a clearer picture of what's going on:
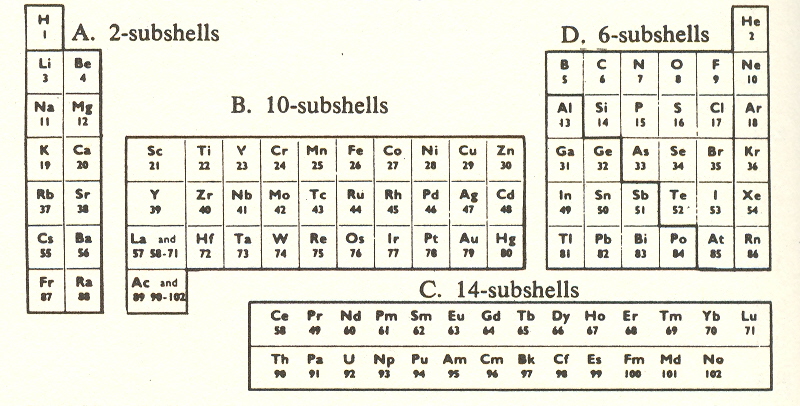
Counting helium as containing the first 2-shell, there are seven 2-shells, five 6-shells, three 10-shells, and one (complete) 14-shell, occurring in nature. (The second 14-shell comprises artificial elements with short lifetimes.)
Build-up of shell structure
We may represent these shells pictorially by taking a typical element from each row. We show below the elements from the second column:
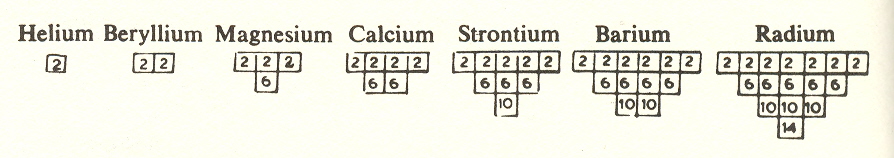
Note the manner in which the sequence builds up progressively:
1. First we have a 2
2. Then another 2
3. Then we have a 6 plus a 2
4. Then another 6 plus a 2
5. Then we have a 10 plus a 6 and a 2
6. Then another 10 plus a 6 and a 2
7. Finally a 14 plus a 10 and a 6 and a 2
The periodic table is trying to tell us something about process. Concerning the atomic kingdom, it says:
Process is cumulative, each stage including what went before. Seven stages of process follow an a-a-b (1-1-2) scheme, each stage repeating before a new development occurs.
Properties of the rows
To discover more, we must draw on the properties which distinguish elements in different rows. These properties are not chemical, at least in the sense that the columns determine chemical properties. But they are quite interesting and suggestive for further study. For this, we need the full-dress version of the periodic table, so that we have complete rows.
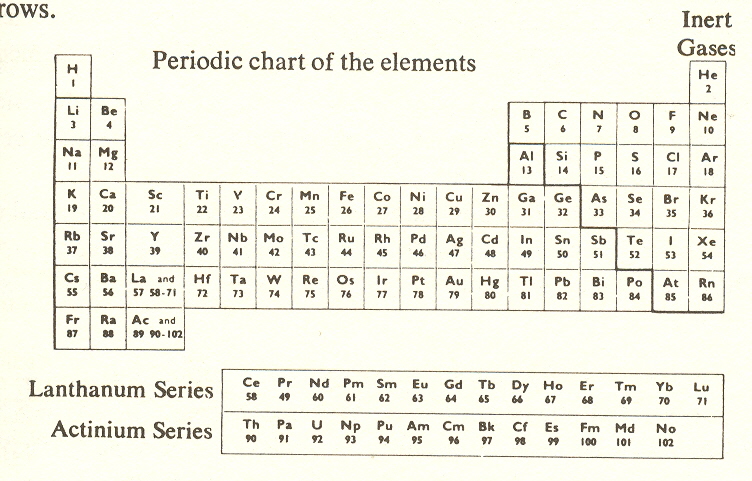
I will now describe the characteristics of the rows of the periodic table in a manner which will later show their correspondence to the seven kingdoms. I.e., the words here in italics characterize the powers of the seven grand stages or kingdoms.
Setting the noble gases (helium, neon, argon, etc.) aside (since they do not form compounds), we note that the first row contains hydrogen. Apart from its predominance as the most common element in the stars, hydrogen atoms are more than twice as abundant as any other element in the human body, and thus hydrogen is the most basic element, like the light that started it all.
The second row contains carbon, oxygen, and nitrogen, which, with hydrogen, are the elements which make up the carbohydrates, fats, and the bulk of the proteins. In other words, the second-row elements make up the substance of the body. These molecules are the building material and the fuel. They have no fixed character, no identity.
The third-row elements contrast sharply with this. They are elements which make molecules that have a special purpose and retain their identity. We can discover this by considering each in turn:
Sodium. Remains as sodium chloride, which functions to maintain sodium-potassium balance.
Magnesium. Outstanding in its special significance as the core atom of chlorophyll molecules.
Aluminum. Not present in the body.
Silicon. Important in shells of diatoms, calamites.
Phosphorus. Makes the DNA double helix; each helix is a row of phosphorus atoms (see p. 80). Also essential to ATP (adenosine triphosphate, a compound which transfers energy and is very important to metabolism).
Sulphur. Important as the "hook" that joins protein chains, and thus makes possible the elaborate molecular structure of the proteins.
Chlorine. Linked with sodium-potassium balance and other special functions.
The fourth-row elements also have special functions, but here it would appear that the combining motif is emphasized. The combining power of iron with the other chemically similar metals in the 10-shell group is important in alloys of steel, which are obtained by addition to iron of chromium, vanadium, cobalt, nickel, etc. Similarly, zinc and copper make the important alloy bronze; nickel and chromium are used for resistive elements in heaters, etc. For the most part, however, we have to turn to the chemistry of life to discover how the latent potentials of the different rows manifest. For example, in the hemoglobin of the blood, iron combines with oxygen at the lungs, which is given up at the muscles.
Of the fifth-row elements, the only one I've found that is essential to the human body is iodine. It so happens that iodine is essential to the thyroid gland, which regulates growth.
Of the sixth-row elements, I can only offer that gold is used in the treatment of arthritis, and hence pertains to mobility; but I feel that this example will not be convincing to the reader.
When we come to the seventh row, chemistry is transcended by radioactive tracer elements. Perhaps man, with the trail of unnatural pollutants he leaves behind, has some resemblance to these seventh-row elements, all of which are radioactive, and destructive to their environment.
Arc-like descent of atoms
We would expect to find some sort of descent in atoms, followed by an ascent. How is this manifested? The descent appears in the curve that represents binding energy in the nucleus of atoms.
While this arc seems rather mild in its gentle contour, it packs a lot of wallop, for the difference between uranium and iron provides the energy release in the atom bomb (fission), and the difference between hydrogen and iron provides that of the hydrogen bomb (fusion). Also, iron is displaced to the left because there are more elements in the rows after iron (on the right). These factors adjusted, we would have a diagram that resembles the are, and even suggests the evolution of the heavier elements from iron, still but partially understood, but thought to have occurred before the present sun existed.
To summarize: we have emphasized the degree to which the atomic kingdom can be rationally explained,; how the variety of atoms is due to the number of proton-electron pairs constituting the respective atoms; and how the periodicity of this number, which creates the rows of the periodic table, divides the one-hundred-odd atoms into seven substages exhibiting, to some extent at least, the properties of the grand stages of process.