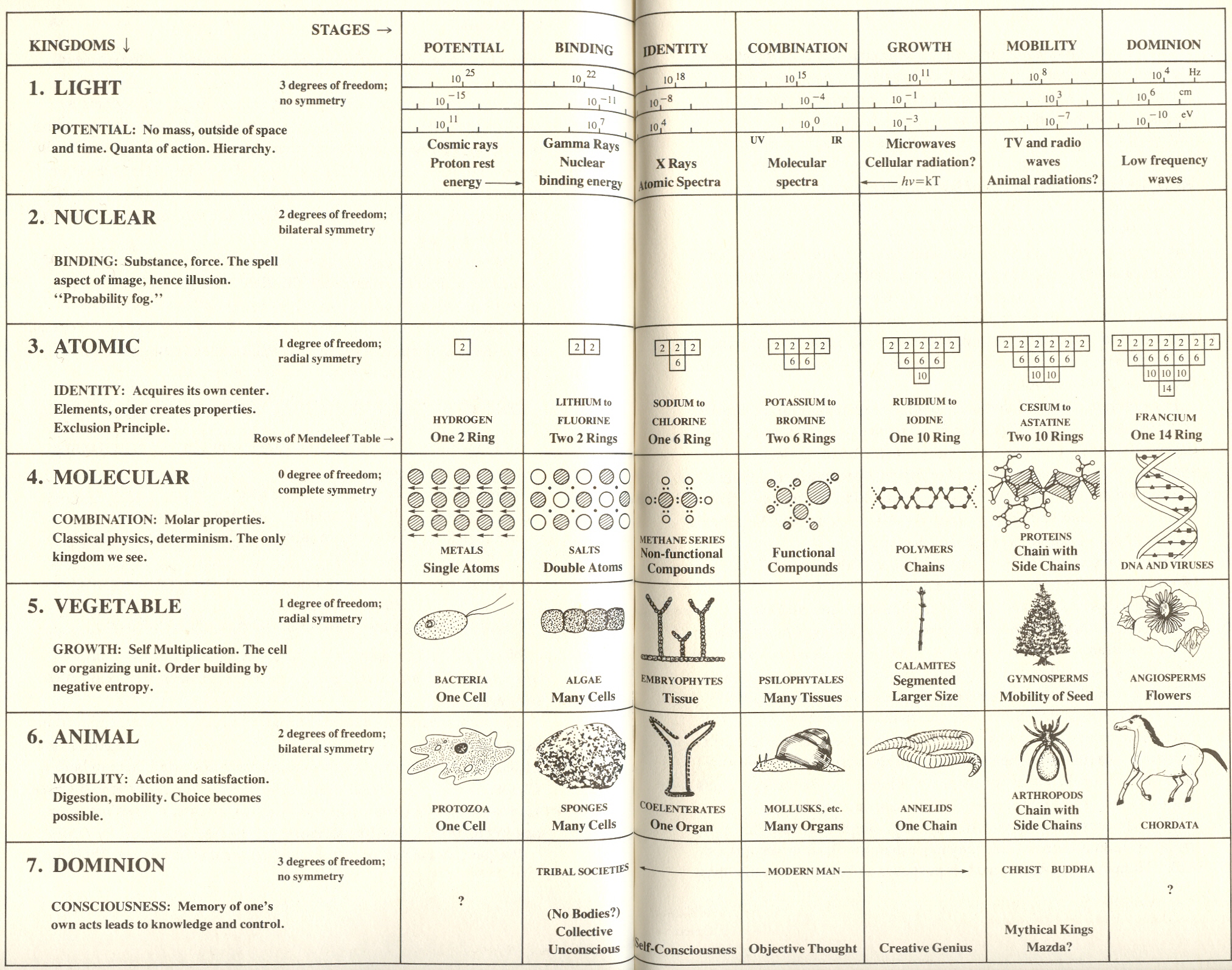
*
THE MOLECULAR KINGDOM
Divisions of chemicals
Whereas the atom organizes proton-electron pairs, the molecule combines atoms. The distinction is helpful and will be clarified in what follows.
There are only about one hundred kinds of atoms; there are countless kinds of molecules. Atoms as atoms exist only singly; when they combine, they form molecules. When molecules combine, the result is another molecule. If we make atoms analogous to the letters of the alphabet, then molecules, being combinations of atoms, are analogous to words. Atoms, like letters, are limited in number; molecules, like words, may be constructed endlessly.
Having found seven kinds of shell in atoms - a distinction on which the seven rows of the periodic table are based - can we find a sevenfold division of the molecular kingdom?
A number of years ago I asked this question of an outstanding chemist, Dr. Charles Price.* He supplied me with the following breakdown of the molecular kingdom into seven substages:
*Personal communication.
1. Monatomic: metals.
2. Ionic compounds: salts, most acids and bases.
3. Nonfunctional compounds: the paraffin series.
4. Functional compounds: compounds of compounds.
5. Nonfunctional polymers: chains 100,000 units long.
6. Functional polymers: proteins, which are chains with side chains.
7. DNA and virus: double helix, self-replicating.
In retrospect, it is surprising how pertinent Dr. Price's breakdown has been, for I have not had to change it despite new ideas, and moreover I've learned from it ideas applicable to other areas of process.
I have found that this kingdom is very helpful to the study of process because it is more completely understood by science than other kingdoms.
These seven broad divisions of chemicals, like the rows of the periodic table, also have characteristics which illustrate those of the seven kingdoms. As we pass from salts (with two atoms) to DNA, with hundreds of millions of atoms organized into a single molecule, we find evidence for an extraordinary evolution. In what follows, I will summarize the basic plot of (this chemical) evolution.
First substage - metals
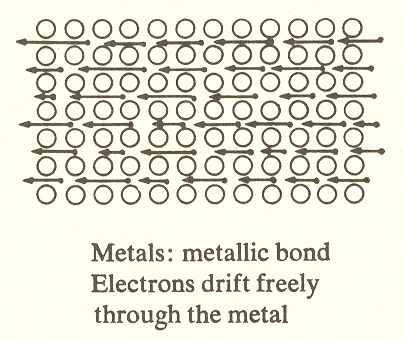
The essence of the molecule is not the constituent atoms, but the bond which holds the atoms together. For the simplest molecules, metals, which are monatomic, the metallic bond leaves the electron free to course through the body of the metal so that it can conduct electricity, as in the case of the telephone or power line. This conductivity of metals is appropriate to the first substage, for it resembles and includes the propagation of electromagnetic radiation at the first stage. (The metal wire is a wave guide for the signal.)

One might wonder why metals, in which the molecules are monatomic, are "molecular" at all; why are they not just atoms? Here we must recognize that the combination of atoms, of which this is the simplest type, results in something other than atoms. It produces metals which have properties that do not exist in single atoms. For example, density, malleability, melting point, conductivity, strength, etc., none of these "molar" (i.e., molecular) properties exists in single atoms.
Second substage - salts
Next in order of complexity are compounds of the simplest sort, such as NaCl (salt), which consists of two kinds of atoms, a positive one (sodium) and a negative one (chlorine), held together by their opposite charges. These positive and negative atoms resemble the second stage, protons and electrons with opposite charges. Salts are soluble in water and form readily into crystals in which atoms are so closely surrounded by their neighbors that it is impossible to say which atom goes with which to make a molecule. The order is such that each sodium is surrounded by six chlorines and each chlorine is surrounded by six sodiums. Thus we can say that this molecule of the second substage, having no identity, is bound to its neighbor in a collectivity.
Let us note the appropriateness of the term "binding" for the second substage. Here one atom gives up one of its electrons to the other. The former acquires a positive charge and the latter a negative charge. In this charged state the atoms are known as ions. The bond in this case is ionic; it comes about because of the attraction which these oppositely charged ions have for one another. But this attraction is communal each positive atom in the crystal is surrounded by negative neighbors, and each negative by positive neighbors, hence binding the whole into a crystal. There is thus no identity to these molecules.
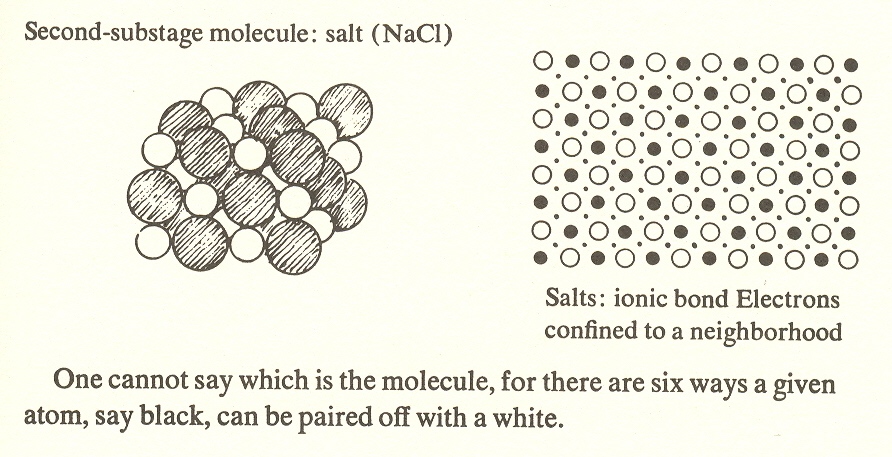
One cannot say which is the molecule, for there are six ways a given atom, say black, can be paired off with a white.
Third substage - methane series
Identity of the molecule is the next development. This innovation is due to a different kind of bond, the covalent bond, which does not depend on attraction. Instead, combining atoms share two electrons, creating a relatively permanent unit. These molecules constitute a sort of alphabet (like the atoms which constitute stage three) . Crude oil is like an alphabet soup of these elemental substances which are sorted into what is known as the methane series by the process of refining. Typical molecules of this series consist of one or more carbon atoms combined with hydrogen.
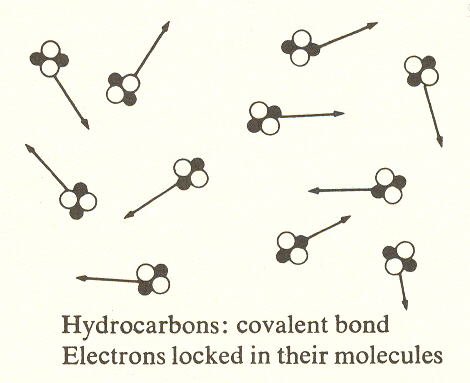
What is important to our philosophic overview is not their contribution to our economy (gasoline, etc.), but that they comprise an ordered set of molecular units which combine with other molecular units to form the great variety of organic compounds of substage four, much as the twenty-six letters combine to form the enormous vocabulary of words.
Additional information
The
simplest example of the covalent bond is hydrogen itself, which occurs
naturally
as molecular hydrogen, ![]() with two hydrogen atoms sharing two orbital
electrons.
with two hydrogen atoms sharing two orbital
electrons.
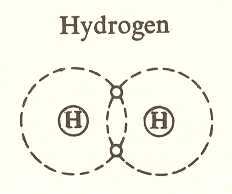
While not a hydrocarbon, because there is no carbon, this molecule could be considered the zeroth member of the hydrocarbon series (see below). It is the simplest covalent bond.
This bond differs from the ionic in giving the molecule an identity which the ionic bond does not have. The ionic bond depends on attraction, and this attraction is felt by the neighboring atoms, resulting in the bonding of many atoms. The covalent bond, like a marriage, binds the partners to one another, making the partnership a unit with relative permanence.
It is this characteristic permanence, or resistance to disassociation, that leads to the term "nonfunctional compounds."
Oils are an example; they do not dissolve in water, and they do not readily combine with other molecules.
The
methane series, an important third-substage group, consists of
combinations of carbon and hydrogen according to the general formula ![]()
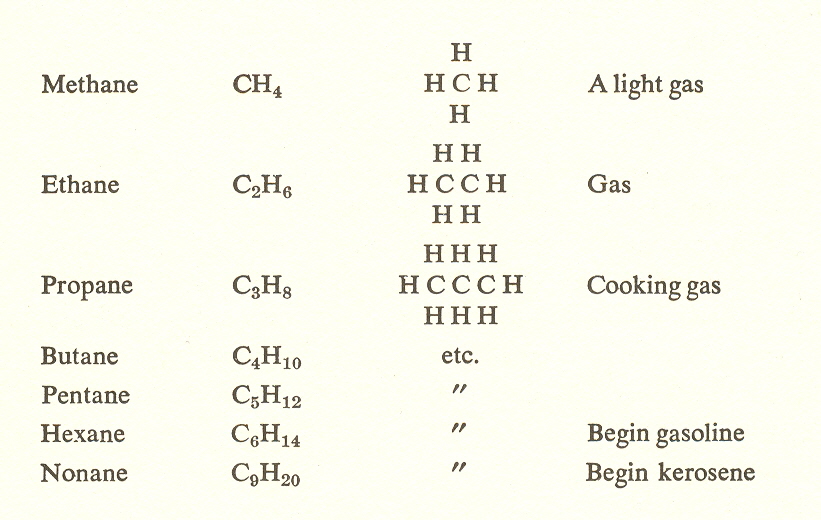
The series adds more and more units of two hydrogen and one carbon until we get fuel oil, lubricating oil, and finally asphalt. This series is also called the paraffin series. The word "paraffin" is derived from "parum" (too little) plus "affiois" (akin), in allusion to its chemical inactivity.
Like atoms, the members of the methane series derive their properties from their position in the sequence; thus the volatility which is desirable in gasoline, and the inertness of asphalt are properties which depend on position in the sequence.
The
series based on the benzene ring is also third-substage. It has the
general
formula ![]() and consists of carbons in a hexagonal ring at
each vertex of which are hydrogens or hydrocarbons.
and consists of carbons in a hexagonal ring at
each vertex of which are hydrogens or hydrocarbons.
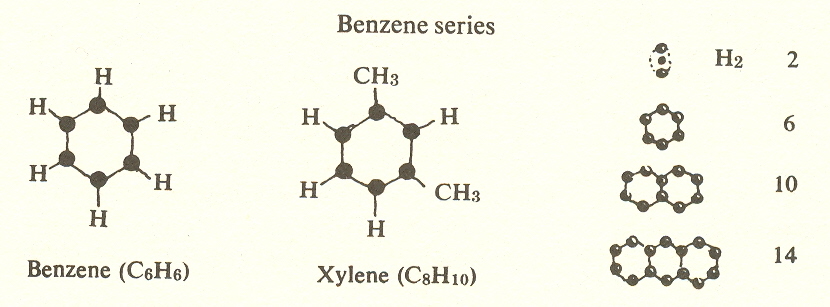
Xylene with two methyl groups has three forms depending on whether the methyls are adjacent or separated by one or by two hydrogens.
Other hydrocarbons combine two or more benzene rings, and it is worth noting that the number of positions in a ring follows the same sequence that electrons do in atoms: 2, 6, 10, and 14.
Fourth substage - functional compounds
Combining the members of the methane series (the alphabet) with radicals (other kinds of letters) creates the functional compounds. Hundreds of thousands of kinds of organic molecule become possible through this combination of combinations. For example, wood alcohol is made from methane plus the OH group; grain alcohol from ethane plus the OH group; and ketones, esters, and amines are derived from other groups. Other alphabets, such as the benzene series, make many more complex molecules.
Additional information
The fourth substage introduces the complexities of organic chemistry and the endless varieties of compounds. So let us not get in too deep. The compounds of the paraffin series we have just considered are nonfunctional; they do not readily associate with other molecules. But if we replace a hydrogen in one of that series, say ethane, by an OH radical (or group), we obtain an alcohol, ethyl alcohol, which is notorious as a good mixer.
What has happened? Alcohol is soluble in water; its contribution is to reintroduce the ionic bond* and communal attraction. The married couples meet at a cocktail party and are attracted to one another; all sorts of combinations result.
*In the form of the hydrogen bond, a weak ionic bond. The weakness of the hydrogen bond is one of the factors that makes life possible, because hydrogen combines at room temperature.
There are other alcohols. Not only does each of the methane series produce an alcohol - methane produces methyl or wood alcohol; ethane, grain alcohol; propane, propyl alcohol; and so on - but we can have alcohols in which two or more hydrogens are replaced by OH radicals. Such is diethylene glycol, used as antifreeze under the trade name of Prestone.
And
there are other radicals. Thus the aldehyde radical, CHO, leads to a whole
series of aldehydes, beginning with formaldehyde; the ketone radical, CO,
creates the solvents used in lacquers and nail polish. The amines, derived
from ammonia ![]() are obtained similarly. The whole series of carbolic
acids found in soap descends from the substitution of COOH for hydrogen in
various members of the methane series.
are obtained similarly. The whole series of carbolic
acids found in soap descends from the substitution of COOH for hydrogen in
various members of the methane series.
Even more interesting are the esters which are responsible for the flavors and odors of fruits and flowers. An example is isoamyl acetate, or banana oil, formed from amyl alcohol and acetic acid.
So far we have listed only the compounds which derive from the methane series. Many more, including the sex hormones, derive from the benzene series. This should suffice to describe the character of the fourth substage, all of which are compounds of compounds.
We have emphasized especially organic compounds because of their importance to life, but minerals that cannot be classified otherwise, as salts, etc., may also qualify as fourth-substage.
Fifth substage - polymers
What advance could be made beyond this combination of combinations? We find it exemplified in the polymers, which consist of chains of hundreds of thousands of units, each unit being made up of some dozen or more atoms. Cellulose, rubber, rayon, and nylon are all examples of polymers. While the structure of the polymer may seem monotonous, the repeated links in the chain are the evolutionary jump which make possible greater wonders to come. Polymers resemble plants not only in producing a chain of cells, but in their capacity to grow. All chemicals from polymers on share this power.
Additional information
Polymers are chain molecules, with hundreds of thousands of identical links. The designation "nonfunctional" refers to their chemical inertness, a consequence of the covalent bond which comes in again here in confirmation of their placement at level III. Another property that marks them as fifth-substage is that they grow, as do cells, in a chain or series of links.
The analogy which likens atoms to letters, and molecules to words, could be extended to make plants analogous to sentences. The life of plants, like the meaning of sentences, is this emergent factor. Applied to polymers, the life-like factor is their ability to store order by drawing energy from the environment. This is an "uphill" manifestation; it goes against the second law of thermodynamics, which decrees that entropy (see beginning of Chapter VIII) is positive. Thus polymers have negative entropy, which is a reminder that the turn has occurred. It is worth noting that the wood of trees consists of polymers, cellulose and lignin, i.e., the fifth stage depends on fifth-substage chemicals.
A conjecture at this writing, still not proved, is that the links of the nonfunctional polymers are fivefold, that is, the number of atoms in a link is a multiple of five.
Sixth substage - proteins
Beyond the polymers in order of complexity are the proteins, which are chains with side chains. The side chains provide means to twist the chain into one of several kinds of helix, and may also hook up to other points on the chain, thus making possible molecules which have an elaborate spatial structure, such as the hemoglobin in blood, which makes a container for picking up oxygen at the lungs and transporting it to the muscles. Blood, hair, nails, skin, horns are all proteins. Thousands of kinds are needed for the animal organism. This multiplicity of shapes and structures of proteins, their mobility and their role in digestion, suggest a resemblance to the sixth or animal kingdom.
We can even see in this protein chain with side chains a foreshadowing of the segmented animal body with its articulated feet - the arthropod.
Additional Information
The proteins are the functional polymers in Dr. Price's list at the beginning of this chapter.
With proteins, the world of molecules acquires magical powers. Impressive as is the contribution of polymers to the structure of trees, we have here an even greater marvel, the possibility of animated substance. For example, actin and myosin. These proteins, which constitute muscle, construct organized battalions of molecules interlaced with veins and arteries (to supply fuel and remove waste) and with nerves (to control). Actin and myosin constitute a chemical that moves.
I will never forget the surprise I received when, having anticipated the existence of a mobile chemical, but not really believing that it existed, I was told that there are indeed mobile molecules, and that muscle, constructed molecule by molecule, is a living structure, one that can move by shortening and lengthening itself.
It is also impressive that a molecule can anticipate the mobility we would expect only of animals. And the movement is not the random motion of particles, such as electrons; this is a coordinated movement of billions of molecules. In fact, recognizing that the bulk of the animal is muscle, we may say that the muscle is "the word made flesh," the vital principle serving the will.
Albert Szent-Gyorgyi discovered that the long actin and myosin molecules overlap their side chains, interlocking like oars of racing shells placed side by side. Activation of the muscle causes the side chains to swing, or shrink, in such a way as to draw the actin and myosin together, thus shortening the muscle.
The more inert or structural proteins include the keratins: skin, hair, fur, wool, horns, nails, claws, hoofs, scales, beaks, and feathers.
Other proteins, including enzymes, are functional. Lysozyme, a chain of 129 amino acids, destroys bacteria by wrapping itself around the molecule in the wall of the bacterium and pulling it apart, a sort of animated monkey wrench like the bewitched broom in "The Sorcerer's Apprentice."
A few proteins have been analyzed. Many years of research were required by Sanger and his collaborators to establish the structure of insulin, which was found to have a molecular weight of 12,000 and to consist of four polypeptide chains, two with twenty-one amino acids and two with thirty. (The amino acids are the side chains referred to above.)
Hemoglobin, which transports oxygen in the blood, is a protein built on a core of four iron atoms. It is estimated that man requires some 50,000 different proteins.
The structure of protein was discovered by Linus Pauling and is called a polypeptide chain; it consists of a chain of links.
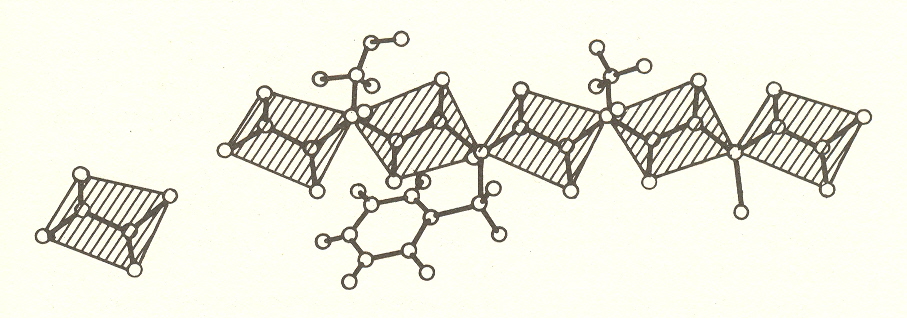
The units of the chain, known as polypeptide links, each consist of six atoms held rigidly in a plane, with carbon atoms at each end joining the links. To each of these joining carbons is attached a side chain, one of twenty amino acids having different structures and different chemical properties. One function of these side chains is to twist the links into a prescribed angle. This causes the chain to form a helix, or, when alternate links are rotated 180º, a ribbon. In both cases, the open comers are joined by hydrogen bonds (a form of ionic bond). There are three possible helices of different pitch, which may be left or right.
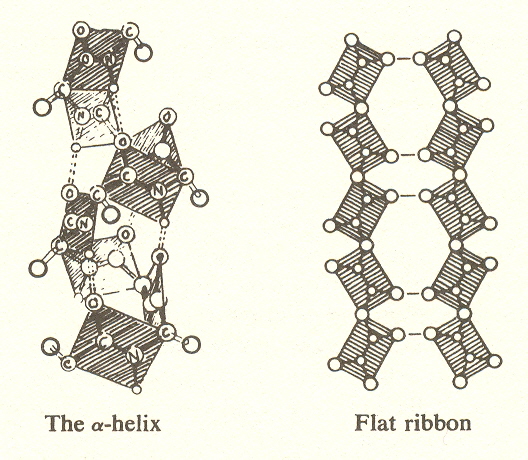
The structural proteins mentioned, hair, skin, etc., consist of helical chains. Silk consists of the flat ribbon.
The functional proteins are more complicated. They include side chains which cause the helix to bend to form a comer at predetermined points, thus making possible molecules such as the hemoglobin of blood, which is a container for the oxygen-carrying iron atom. Other functional proteins such as enzymes make miniature laboratories in which particular molecules can be synthesized or broken down.
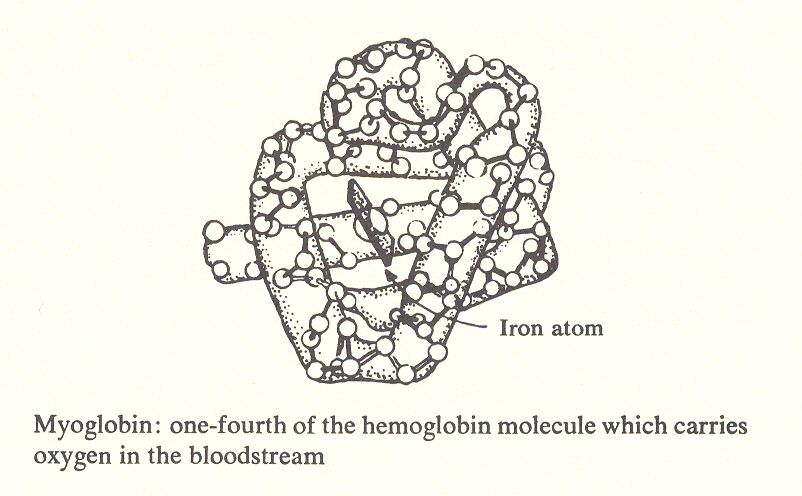
Thus the linked peptide units, together with the side chains that determine their angle, comprise a sort of construction set like the mecanno toy which makes possible a huge variety of shapes. Still I find it incredible how even this ingenuity can construct a feather.
To sum up, the functional polymers or proteins at substage six add a much greater versatility to the growth power of the previous substage. This versatility, in the functional polymers especially, becomes a kind of animation, a mobility. Further study is needed, but it appears that the extra range of behavior, or freedom, of the proteins is attributable to the ionic bond which reenters the picture at the sixth substage. Recall that the fifth substage restores the covalent bond, which holds the links of the chain together. In proteins, it is the ionic bonds of the side chains that provide the versatility of helices and different bonds, not to mention the contraction of the side chains which join actin and myosin. The ionic bond is the sexy one, even as it was at substage two where the sodium atom gives its electron to fill the hole in the chlorine shell.
A
final note: according to our arc, the sixth-substage bond should have two
degrees of freedom. It is clear from the variety here possible that the
bond is much more versatile than in the fifth substage, but I have only
recently noted the feature of the peptide links that makes the two degrees
of freedom precise. This is that the peptide links can be twisted with
respect to one another through two angles ![]() , and
, and
![]()
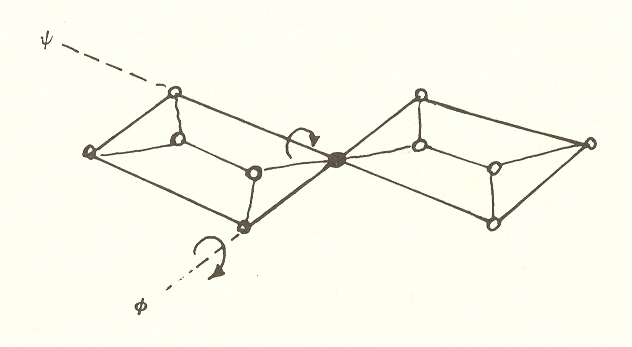
It is these two angles, each a degree of freedom, that make possible the variety of structures and confirm the prediction of two degrees of freedom.
Note too the increasing homogeneity at this substage. All proteins are polypeptide chains, whereas the fifth-substage polymers came in great variety.
Seventh substage - DNA
Recently discovered by Watson and Crick, DNA consists of a double helix whose ladder-like rungs code the information necessary for the cell to duplicate itself or for it to build a multicellular organism. All DNA is of the same external shape in contrast to the different shapes of the proteins it controls. This fact would seem to demonstrate the same principle which may underlie the fact that man has a singular form while animals take so many shapes. As the DNA (in the seventh substage) carries information and manufactures the proteins that do the work, so man (in the seventh stage) makes the tools he needs and uses the animals he domesticates to do his work.
Additional information
Prior to the discovery of DNA in 1953, biologists for some time had recognized that cellular processes, including cell division and inheritance, are governed by particles, visible under the microscope, which they called chromosomes. Watson and Crick made the breakthrough that established that chromosomes ultimately consist of DNA molecules. They found DNA to be made up of a double helix, two chains of phosphates linked like a ladder by rungs joining the two helices together. The rungs are of four different kinds which, taken in groups of three, provide a twenty-letter alphabet. much as dots and dashes make up the Morse code.
Each of the twenty "letters" of this alphabet designates one of the twenty amino acids which make up the proteins discussed in the preceding section on the sixth substage.
Thus the DNA spells out a message which describes the exact sequence of amino acids required for manufacture of the particular proteins which in turn build the organism. While it is not known how the timing of the manufacture of the thousands of necessary proteins is achieved, it is known that the molecular weight of DNA in one of the simplest organisms, Escherichia coli, is over 3 billion (3 billion protein-electron pairs). This would allow for at least 6 million letters in the message (allowing for fifty atoms of an average molecular weight of 10 for each rung of the ladder). This amount of information would require a two-thousand-page volume. DNA therefore can be regarded as a kind of blueprint, coding instructions for manufacture.
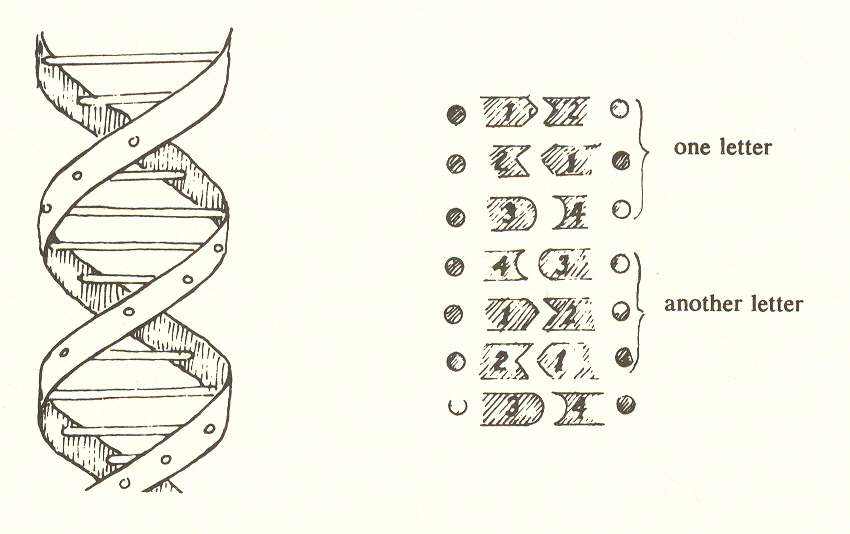
It is significant for our theory that the culmination of molecular evolution is a single species of molecule - DNA. We may recall that the protein molecules (substage six), while all built on the polypeptide chain, have many shapes: the strands of fibrous protein for hair, the complex cage of hemoglobin, the interlocking batteries of actin and myosin for muscle. With DNA we have but one shape, the double helix, whose variety is not in its outward form, but in the information it encodes. If we liken the proteins to different kinds of tools, the DNA could be likened to a book - its external shape gives no clue to its content. This uniformity of shape of all DNA molecules is an expression of the homogeneity we referred to in Chapter IV as characterizing photons. This homogeneity is an expression of the top level, the "intelligible" world of light, in this case because of the encoding of pure information. Molecular evolution ultimately reenters the "intelligible" world of light, in this case by encoding pure information.
This brings into focus another fact about DNA, which is that while it encodes the instructions and dictates manufacture, it does not itself do the work; it delegates this function to the so-called messenger RNA which, put together by the DNA, goes out into the cytoplasm (the body of the cell) and manufactures proteins. This aloofness from "manual" exercise helps us to recognize that even here, at the molecular level, there is a distinction between the governing or control principle and the manufacture itself.
We are thus led to recognize the key word "dominion" as suitable for the seventh substage (see p. xxiii).
The symmetry, or lack of it, that we expect at the seventh substage is also borne out, for the double helix, being a spiral, lacks radial symmetry, and, being different along its length, lacks axial symmetry. This lack of symmetry correlates with the complete dedication of DNA to meaning; it is a message with no flourishes (see Chapter IV).
Because it is made up of DNA, we should include the virus as seventhsubstage. Virus, in fact, is almost pure DNA, for it lacks the other substances contained in living cells that make possible reproduction. To obtain these substances, the virus invades the cells of plants or animals and "changes the blueprints" of the host DNA, to force it to make more virus. So the virus is a sort of desperado or highjacker, whose takeover of the cell is a clear-cut example of dominion. In fact, it was the virus that suggested to me the power of the seventh stage in general. While we can condemn its behavior, we owe our existence to the similar capability of our own DNA, for without it, when we eat chicken, we would turn into a chicken.
Let us now take a closer look at the structure of DNA. The cross links or rungs of the ladder consist of four nucleotides which join as pairs; cytosine to guanine and adenine to thymine.
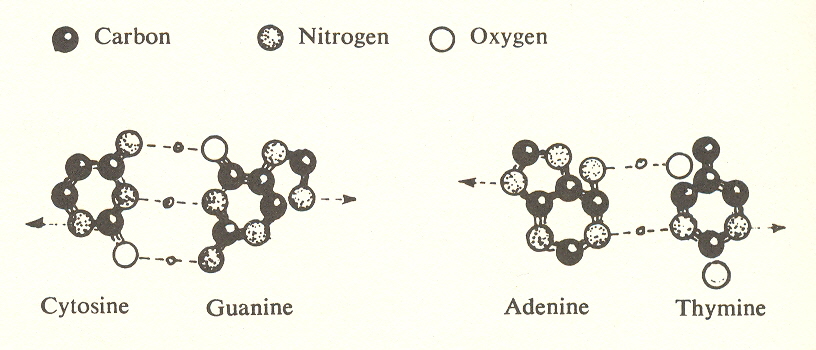
Each pair can be reversed end to end, making four possibilities: CG, AT, GC, and TA. The end of each pair joins to a pentose sugar, and this in turn to a phosphorus atom (together with its four oxygens).
When the whole molecule splits, each half retains a complete complement of half units which prescribe their mates on the new chain-to-be, making it possible for each half to duplicate its matching partner and become a new double helix. If you think this is complicated, read Watson's The Double Helix.* It was his recognition that complementary, not similar, pairs went together that solved the mystery of DNA and led to Watson's receiving the Nobel Prize.
*Watson, James. The Double Helix: Being a Personal Account of the Structure of DNA. New York: Atheneum, 1968.
One further point is of speculative interest: counting guanine and adenine, each of which contains a five- and six-ring as two units, and counting along a rung from one end to the other, we find there are seven elements on each rung of the DNA ladder.
While the significance of this is unknown, it fills in the picture of an ascending complexity in the molecules of each substage. (The peptide units were sixfold.)
This kind of conjecture, except for the periodic table itself, is not a part of current chemistry, but is promising as a speculative device. There is certainly an increasing complexity as we go through the levels of organization of molecules; and aside from the mere count of the number of atoms in a molecule, or even of the kinds of atom required, there is the possibility that the numbers from one to seven can distinguish kinds of molecular topology, and therefore indicate a more profound relationship than is recognized in current science. (See Appendix II.)
Correspondences on the arc
To return to the molecular kingdom as a whole, we can now appreciate that the development that has taken place from the metals to DNA is itself a process.
If we arrange these substages in an arc, we discover something of great interest.
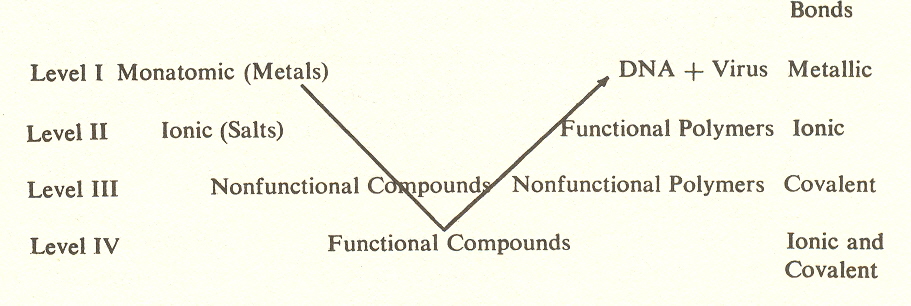
When we examine the type of bond which prevails in each substage, we find substage four combines ionic and covalent bonds, substages three and five are covalent, substages two and six are ionic. Since the monatomic molecules are metals, substage one is, of course, metallic bond. We predict the same for DNA, which seems likely in view of its probably superconductive core. (This suggests the possibility that the DNA radiates a short-wave radio signal which coordinates cell growth.) Less speculative is the difference in freedom of the bonds on the successive levels. At level I the bonding electrons of metals move freely through the body of the metal, that is, the bond is unlocalized. At level II the electrons of ionic compounds create bonds which connect each atom with its neighbors. And at level III the electrons of covalent compounds are confined to the molecule itself.* (See earlier illustration.)
Correspondences of substages to kingdoms
Not only are there seven substages, but each substage has a similarity to the corresponding kingdom:
1. The conductivity of metals, with their free-ranging electrons, corresponds to electromagnetic radiation in the kingdom of light.
2. The binding of ionic compounds, with their positively and negatively charged atoms, corresponds to the proton and electron, the positive and negative particles.
3. The identity of the hydrocarbons in the paraffin series corresponds to atoms, which also form an "alphabetical" sequence with properties determined by their position therein.
4. The combinations which we call functional compounds are fourth-substage because they are combinations of combinations.
5. The polymers in their self-replication and chains of units correspond to plant growth with its cell division.
6. The proteins, as the chemicals that move, correspond to the animal kingdom.
7. DNA, which directs and governs the growth of plants and animals, gives us the clue to the distinctive character of the seventh kingdom; we can now call it the dominion kingdom.
Dependence of seventh stages and substages
on what is beyond themselves
Note that we have defined the dominion kingdom (seventh stage) from evidence drawn from the seventh substage, DNA and virus.
Both DNA and virus are boss molecules which direct the activity of the cell "factory" and hence warrant the dominion designation - but there is an interesting additional point - that is: that the seventh substage, DNA and virus, requires cells for the completion of its function; and cells belong in the next higher kingdom. It will be found that all seventh substages require the next higher kingdom to function. For example, the flowering plants depend on insects for pollination. Equally remarkable is the dependence of controlled radioactivity of atoms (third stage) on molar concentration (fourth stage). It was once thought that radioactive disintegration could not be influenced by any external factor, but the atom bomb is evidence to the contrary.
Both the atom bomb and the atomic pile depend on bringing together enough uranium atoms so that the disintegration products of one atom (neutrons) will cause the disintegration of other atoms. In the bomb this process proceeds instantaneously, causing an explosion. In the pile the process is moderated or slowed down by carbon rods pushed between the atoms to absorb the radiated neutrons and control the rate of fission.
What would this mean to the dominion kingdom? Since it is already the highest kingdom for the solar universe, we are led by this and other evidence to expect yet higher stages - a super arc which deals in galactic evolution. In other words, the dominion kingdom requires something beyond itself, which may help to explain why all human cultures, with the possible exception of modern man, depend on a belief in higher orders of beings, gods.
This dependence of seventh substages on the next higher stage is one of the most difficult concepts to accept because it suggests that process, at the seventh substage at least, anticipates its own future. Despite this seeming incredibility, I think we should try to heed this point, for the evidence presented in the substages is confirmed by the theory. That is, the phenomena suggest a recurrence of the teleology implied by the behavior of light, whose capacity to take the shortest path to its goal so impressed Leibniz and Planck.
Powers of the kingdoms
We designate below the key word or power for each kingdom (stage):
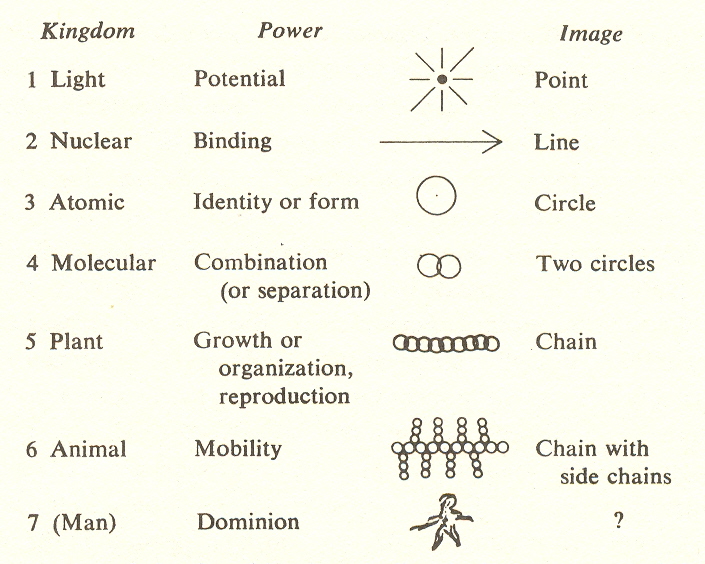
The power of the first kingdom is listed above as potential. This designation is not entirely justified by the corresponding molecular substage, but we will show the appropriateness of this choice in later development.
A final point about the molecular kingdom, sufficiently arresting to deserve comment: the kingdom starts by creating the properties we associate with matter - density, hardness, strength, conductivity, boiling point, etc. These are the properties which emerge when millions of atoms are brought together. As the power of combination develops, these properties undergo a subtle change. What seems to occur is that the individual molecule acquires a kind of responsibility: it extends its domain more and more, until it is not only itself organized from millions of atoms, but (as DNA) it builds an organism billions, trillions of times its own size.
The grid
On the basis of our survey of the atomic and molecular kingdoms, we can now put forward a scheme which divides each of the seven kingdoms into substages. Each substage contributes, to the kingdom of which it is a part, a thrust or power similar to the thrust or power which the numerically corresponding kingdom makes to the whole of process.
Referring to the grid: the left-hand column lists the kingdoms by number and name. Below each name is the word for the corresponding power, together with other words that characterize the kingdom. In the upper right-hand comer of the rectangle of each kingdom is its degree of freedom and symmetry. The next seven narrow columns describe the substages. Note that the key word for each substage is the same as that for the power of the kingdom.
The top row of the grid divides the light kingdom into substages by frequency (CPS) beginning with the highest frequency (or greatest energy) at substage one. Thereafter each substage begins with a frequency approximately 1/2000 as great. Wave length is shown in cm., and energy in electron volts. So divided, each substage includes the frequencies that activate the corresponding kingdom.
Substage one: Cosmic rays - high energy particles of more than 1 G.E.V.
Substage two: Photons that create protons to electrons, gamma rays
Substage Three: X rays, atomic spectra
Substage Four: Visible radiation and molecular spectra
Substage
Five: Microwaves about ![]() to 10 cm. (predicted for cells)
to 10 cm. (predicted for cells)
Substage Six: Shortwave radio 100 cm. to 10 meter (predicted for animals)
Substage seven: Long-wave radio 10 meter to 20000 meter
Note that visible light is in the exact center and that room temperature (at which life begins) comes at the beginning of the fifth substage.
The substages of the nuclear kingdom have not yet been assigned in satisfactory fashion and are left blank. The substages of the atomic and molecular kingdoms are set forth as we have discussed them in both Chapter VI and this chapter. The substages of the vegetable and animal kingdoms will be covered in the next two chapters. The last, or dominion, kingdom will be discussed in the final chapters.
We have discussed the atomic and molecular kingdoms. The vegetable and animal kingdoms will be covered in the next two chapters. The first two kingdoms, light and nuclear particles, are not sufficiently understood to analyze at this time. The last, or dominion, kingdom will be discussed at the end of the book.
Recapitulation and preview of later chapters
The importance of this chapter is that it confirms the thesis of an arc. The development from molecules with but one atom to DNA with billions of atoms is as much an evolution as is the evolution of cellular life from one-celled bacteria to trees and elephants. But because chemistry is a more exact science than is biology, it can provide a basis on which to build; it can give sanction to the conjectures on which we based the arc and elevate them to the principles we need to discover the nature of human evolution.
For example, we noted that the evolution from photons to molecules entailed a loss of freedom followed by its recovery under control. This is true within the molecular kingdom, where the bonding electrons in metals are free to move at the speed of light but become increasingly constrained in salts and completely so in oils, ultimately to regain their freedom in proteins and DNA, where controlled electrons make possible the functions of these highly organized molecules.
Again, we saw how the symmetry of the arc of kingdoms (Chapter IV) manifested for molecules in the similarity of bonds, i.e., ionic bond at substages two and six and covalent at substages three and five.
Most interesting is the loss and subsequent regaining of homogeneity - which is dramatically demonstrated in the progression from polymers with many kinds of chains, to proteins using only the polypeptide chain, and again from the variety of side chains in proteins to four of DNA.
This will be important when we come to man's evolution, for the fact that molecules develop from a sixth substage having a variety of shapes to a seventh having one shape (the double helix) is evidence that the contrast between the variety of shapes in animals and the unity of man's shape (all men are of one species) can be seen as a principle.
This, of course, is only part of the story. Despite anatomical similarity, humans are not alike any more than are DNA molecules, or books. Humans are different in their purposes and character rather than their physical attributes. This has importance for human evolution, which we will take up at the end of the book.
But before treating this important theme, human evolution, we need to explore and describe the two types of evolution which precede the human, the evolution of the cellular organism in the plant kingdom and the evolution of the animals; these are distinct from human evolution. The next chapters will be devoted to this subject.